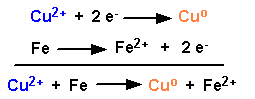
All we know is that this pair of reactions gives 0.78 volts with 1.0 M solutions.
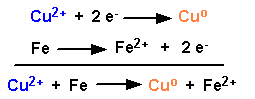
Let's assign a value of 0.00 volts at standard pressure and temperature to this reaction:
![]()
When measured against a standard hydrogen electrode, the potential for the reduction of Cu2+ is found to be 0.34 volts.



(c) Copyright 2000. All rights reserved.