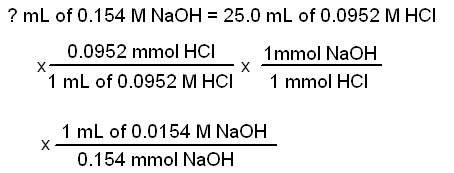|
Example:
What is the concentration of an aqueous solution of hydrochloric acid
if 25.0 mL of the acid reacts with an excess of solid calcium carbonate
to yield 0.307 L carbon dioxide, measured at 0.95 atm and 25°C?
Solution
Analysis of the problem
1. The product of the reaction is a gas whose volume was not measured at standard conditions of temperature and pressure. We can calculate the number of moles of gas produce by using the ideal gas equation: PV = nRT
2. Using the number of moles of carbon dioxide produced, we can calculate
the number of moles of hydrochloric acid used. The mles of HCl are in
25.0 mL solution. Molarity is a ratio between moles of solute and volume
of solution. By dividing the number of moles of HCl by the volume (L)
of solution in which it was dissolved, we will obtain the molarity of
the acid solution.
Calculations
 
2. Carry out the stoichiometric calculation.
equation
2 HCl + CaCO3  CO2 + CaCl2 +H2O CO2 + CaCl2 +H2O
Wanted
The molarity of the acid solution or mol HCl/L solution
Given
0.012 mol CO2
Conversion factor
2 mol HCl yield 1 mol CO2
Arithmetic equation

Answer
0.96 M HCl
|
BaSO4 + 2 HCl
 Answer
Answer