Characteristics
of the Solid, Liquid, and Gaseous States
In Sections 1.3 and 2.5A3,
we noted that the physical properites of a particular substance determine its
state at room temperature. If both its normal melting point and its normal boiling
point are below room temperature (20°C), the substance is a gas under normal
conditions. The normal melting point of oxygen is -218°C; its normal boiling
point is -189°C. Oxygen is a gas at room temperature. If the normal melting
point of a substance is below room temperature, the substance is a liquid at
room temperature. Benzene melts at 6°C and boils at 80°C; it is a liquid
at room temperature. If both the normal melting point and the normal boiling
point are above room temperature, the substance is a solid. Sodium chloride
melts at 801°C and boils at 1413°C. Sodium chloride is a solid under
normal conditions. Figure 9.1 illustrates the relationship between physical
state and normal melting and boiling points.
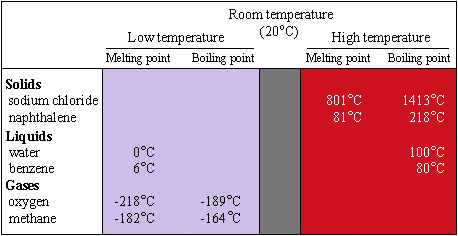
| FIGURE 9.1 The physical state as related to normal melting and boiling points. Notice that the solids melt and boil above room temperature, the liquids melt below room temperature and boil above room temperature, and the gases melt and boil below room temperature.
|
A. Shape and Volume
A solid has a fixed shape and volume that do not change with the shape of its container. Consider a rock and how its size and shape stay the same, regardless of where you put it. A liquid has a constant volume, but its shape conforms to the shape of its container. Consider a sample of milk. Its volume stays the same, whether you put it in a saucer for the cat to drink or in a glass for yourself; clearly its shape changes to match the shape of the container. A gas changes both its shape and volume to conform to the shape and volume of its container. Consider a sample of air. It will fill an empty room, a balloon, a tire, or a rubber raft. Its shape and volume conform to the shape and volume of the container in which it is placed. Figure 9.2 illustrates these points.
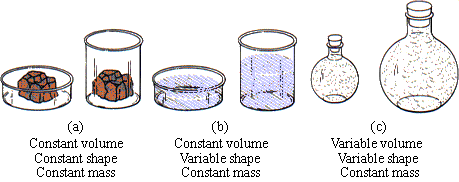
| FIGURE 9.2 Constancy of volume, shape, and mass in the three states of matter: (a) solid, (b) liquid, (c) gas.
|
B.Density
The densities of liquids and solids are measured in grams per milliliter and grams per cubic centimeter, respectively, and change very little as the temperature of the sample changes. Gases have much lower densities, so much lower that gas densities are measured in grams per liter instead of grams per milliliter. The density of a gas varies considerably as the temperature of the gas changes. Table 9.1 shows the densities of three common substances, one in each of the three physical states, at two different temperatures.
TABLE 9.1 Densities of three common substances
|
| Density at 20°C |
Density at 100°C |
| solid: sodium chloride |
2.16 g/cm3 |
2.16 g/cm3 |
| liquid: water |
0.998 g/mL |
0.958 g/mL |
| gas: oxygen |
1.33 g/L |
1.05 g/L |

C. Compressibility
The volume of a solid or a liquid does not change very much with pressure. You
cannot change the volume of a brick by squeezing it, nor can you squeeze one
liter of liquid into a 0.5-L bottle. The volume of a gas does change a great
deal with pressure; you can squeeze a 1.0-L balloon into a 0.5-L space.
D.Inferences about Intermolecular Structure
The constant shape and volume of a solid suggest that its particles (atoms,
ions, or molecules) are held together by fairly rigid bonds. The variable shape
and constant volume of a liquid suggest that there is some bonding between its
particles but that these bonds are not rigid and probably are less strong than
those in a solid. The fact that a gas has neither constant shape nor constant
volume suggests that there are no bonds and only very slight interactive forces
between the particles of a gas. The variety in compressibility suggests other
hypotheses. If solids and liquids cannot be compressed, the particles of which
they are composed must be very close together. The high compressibility of a
gas implies that the particles of a gas are very far apart with a great deal
of space between them. This last hypothesis is supported by the difference between
the densities of solids and liquids and the densities of gases. One mL of a
solid or liquid always has much more mass than does one mL of a gas.
