 |
Chemistry
234
|
Experiment 3: Fractional Distillation (15 points)
Summary
Fractional distillation is a technique used to separate volatile compounds with boiling points that differ by less than 50° C. In this experiment, you will distill a mixture of methanol (b.p. 65°C) and water (b.p. 100°C). Using data obtained in this experiment, you will graphically demonstrate how head temperature changes with the composition of the distillate.
Procedure
Obtain 60 mL of a sample of one of the methanol/water mixtures from the carboys in the lab. Record which sample you chose (A, B, or Q). Add the mixture and a few boiling chips to a clean 100 mL round bottom flask. Set up the apparatus for fractional distillation as demonstrated in lab.
|
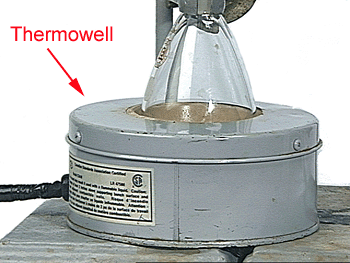
Use the Thermowell to heat the flask. Do not put the Thermowell directly on the desk top because of the heat. |
 |
|
Plug the Thermowell into the Variac on your bench. Do not plug directly in the wall outlet. WARNING: If operated at 110 V without a flask the thermowell can reach 500 - 550 °C. |
 |
| Use a distilling column to help separate the mixture. |  |
| You will use a digital thermometer to measure the distillation temperature. |  |
| The temperature probe is fitted with a thermometer adapter so it fits in the standard taper joint of the distillation head. |
 |
| The thermometer adapter allows you to put the thermometer into the distilling adapter. |  |
| Use joint clamps and hose clamps. | 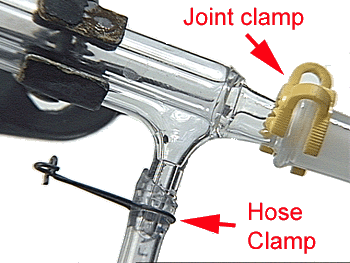 |
| Here is the completed apparatus. |  |
Have your set-up approved by your TA before you begin.
Begin heating the sample on a low setting and increase the temperature as needed. Collect the distillate in a graduated cylinder. Record the head temperature when you collect the first drop of distillate, and then record the head temperature at every two milliliters of distillate collected. Stop the distillation when you have recorded three readings at about 100°C, even if you haven't distilled all of the sample.
DO NOT heat the flask to dryness.
Report
Your report should include the following:
1 . References to procedure with changes noted
2. Table of recorded data (volume collected and head temperature)
3. Graph of distillation data: Make a plot with the head temperature on the y-axis and the volume of distillate on the x-axis (see page 125 of your text). Draw an AVERAGE line through the data points. DO NOT simply connect the dots.
4. Summary table of data: Use your graph to determine the volume and boiling point range of each "fraction." Make a table that includes Fraction number, collected volume, and boiling range. Here is a sample table structure.
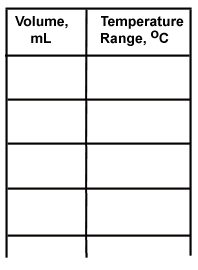
5. Interpretation of data and conclusion, including the methanol/water ratio of your sample.
Answer these questions in your report:
1. Sketch and label the apparatus you used for a fractional distillation.
2. Using figure 4.3 on page 125 of the textbook, if a mixture has 60 mole % toluene and 40 mole % benzene:a. At what temperature will the solution start to boil?
b. What is the composition of the first small amount of vapor that forms?
c. If the vapor formed in question 2b is condensed and the resulting liquid heated to its boiling point what is the composition of the new vapor?
Tutorials
You should work the web-based ChemNet tutorials on distillation before going to the lab.