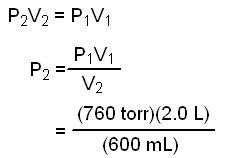
Sample Problem:
An ideal gas occupying a 2.0 L flask at 760 torr is allowed to expand to a volume of 6,000 mL.
Calculate the final pressure in atm.
Let's solve Boyle's law for P2 and then substitute values for P1, V1 and V2.
Ooops! The units are torr-L/mL instead of atm. To fix this problem let's start by converting torr to atm.

