Kinds
of Chemical Changes
One common way of classifying chemical reactions is to separate them into
four categories: combination, decomposition, displacement, and double displacement.
We give several examples of each type for two reasons: (1) to ensure that you
understand the scope of each category and (2) to help you gain experience in
interpreting and balancing equations. You may want to refer back to Section 3.4A. for a list of the
rules and steps to follow in balancing equations.
TABLE 8.1 Parts of a chemical equation
| Reactants |
The substances that combine in the reaction. Formulas must be correct. |
| Products |
The substances that are formed by the reaction. Formulas must be correct. |
 H H |
The enthalpy (heat energy) change accompanying the reaction. Energy
is released if 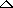 H < 0; energy is absorbed if
H < 0; energy is absorbed if 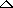 H > 0
H > 0 |
| Arrows |
|

|
Found between reactants and products, means "reacts to form." |
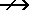
|
Means the equation is not balanced. |

|
Placed after the formula of a product that is a gas. |
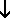
|
Placed after the formula of a product that is an insoluble solid - that
is, a precipate.
|
| Physical state |
Indicates the physical state of the substance whose formula it follows. |
| |
(g) Indicates that the substance is a gas |
| (l) Indicates that the substance is a liquid |
| (s) Indicates that the substance is a solid |
| (aq) Means that the substance is in aqueous
(water) solution |
| Coefficients |
The numbers placed in front of the formulas to balance the equation. |
| Conditions |
Words or symbols placed over the arrow ( )
to indicate conditions used to make the reaction occur. )
to indicate conditions used to make the reaction occur.
|
| |
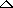 |
Heat is added |
| hv |
Light is added |
| elec |
Electrical energy is added |
|
A. Combination Reactions
In a
two substances combine to form a single compound. Two examples are the reaction of solid magnesium with gaseous oxygen to form magnesium oxide, a solid:
2 Mg(s) + O2(g)  2 MgO(s)
2 MgO(s)
and the reaction of a hydrogen gas with chlorine gas to form gaseous hydrogen chloride:
H2(g) + Cl2(g)  2 HCl(g)
2 HCl(g)

| FIGURE 8.2 The brilliant white light associated with some fireworks is due to the release of energy when magnesium reacts with oxygen. |
Figure 8.2 illustrates an example of a combination reaction.
Other combination reactions have compounds as reactants. The reaction of gaseous carbon dioxide with solid calcium oxide to form solid calcium carbonate is an example of such a reaction.
CaO(s) + CO2(g)  CaCO3(s)
CaCO3(s)
|
Example
Write balanced equations for the following combination reactions:
a. When solid phosphorus, P4, is burned in chlorine gas, solid phosphorus
trichloride is formed.
b. When gaseous dinitrogen pentoxide is bubbled through a solution of
water, nitric acid is formed.
Solution
a. Write the reactants, an arrow, and then the products, with the physical
state of each reactant or product shown after its formula. Write conditions
for the reaction over the arrow.
P4(s) + Cl2(g) 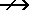 PCl3(s)
PCl3(s)
For atoms of phosphorus will yield four moleucles of phosphorus trichloride, which will require 12 atoms (six molecules) of chlorine. Putting in thesecoefficients gives the balanced equation
P4(s) + 6 Cl2(g)  4
PCl3(s) 4
PCl3(s)
b. Write the reactants, an arrow, and the products.
N2O5(g) + H2O(l) 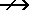 HNO3(aq)
HNO3(aq)
Include the physical states and conditions of the reaction as given in
its statement. Starting with nitrogen (it is always wise to leave hydrogen
and oxygen to the last), two atoms of nitrogen in one molecule of N2O5
will form two molecules of nitric acid. Each molecule of nitric acid contains
one hydrogen atom, thus two molecules will require two hydrogen atoms
or one molecule of water. We then have six atoms of oxygen in the reactants
- the same number of oxygen atoms required by two molecules of nitric
acid. The equation is now balanced:
N2O5(g) + H2(l)  2
HNO3(aq) 2
HNO3(aq)
|
B. Decomposition Reactions
In a decomposition reaction, a compound is decomposed to its component elements
or to other compounds. Although some compounds decompose spontaneously, usually
light or heat is needed to initiate the decomposition. Following are three examples
of decomposition - one induced by light, one induced chemically by a catalyst,
and the third caused by heat. The antiseptic hydrogen peroxide is sold in opaque
brown bottles because hydrogen peroxide decomposes in light (Figure 8.3). The
equation for this decomposition is:
| |
2 H2O2(aq) |
hv
 |
2 H2O(l) + O2(g) |
Oxygen can be prepared by heating solid potassium chlorate in the presence of manganese dioxide, a catalyst. A catalyst is a chemical that, when added to a reaction mixture, hastens the reaction but can be recovered unchanged after the reaction is complete.
| |
2 KClO3(s) |
MnO2
 |
2 KCl(s) + 3 O2(g) |
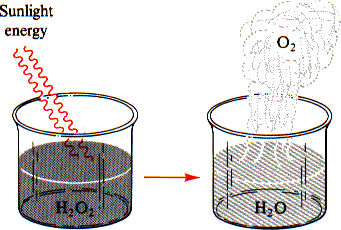
| FIGURE 8.3 Light hastens the decomposition of hydrogen peroxide. The dark bottle in which hydrogen peroxide is usually stored keeps out the light, thus protecting the hydrogen peroxide from decomposition. |
When slaked lime, Ca(OH)2(s), is heated, lime (CaO) and water vapor are produced:
Ca(OH)2(s)  CaO(s) + H2O(g)
CaO(s) + H2O(g)
|
Example
Write balanced equations for the following decomposition reactions:
a. Solid amonium carbonate decomposes at room temperature to ammonia,
carbon dioxide, and water. (Because of the ease of decomposition and the
penetrating odor of ammonia, ammonium carbonate can be used as smelling
salts.)
b. On heating, lead(II) nitrate crystals decompose to yield a solid lead(II)
oxide and the gases oxygen and nitrogen dioxide.
Solution
a. The unbalanced equation for the decomposition of ammonium carbonate
is:
(NH4)2CO3(s) NH3(g)
+ CO2(g) + H2O(l) NH3(g)
+ CO2(g) + H2O(l)
Inspection of this equation indicates that two nitrogen atoms, therefore
two ammonia molecules, are needed on the right. With this change, all
other atoms are balanced:
(NH4)2CO3(s))  2 NH3(g)
+ CO2(g) + H2O(l) 2 NH3(g)
+ CO2(g) + H2O(l)
b. Writing the formulas for the reactant and the products in the form
of an equation gives:.
Pb(NO3)2(s)  PbO(s)
+ O2(g) + NO2(g) PbO(s)
+ O2(g) + NO2(g)
Balance the elements in the order Pb, N, O, to leave oxygen for the last,
which is in general a good practice. The lead is balanced as it stands,
one atom on each side. There are two nitrogen atoms on the left, therefore
we need 2 NO2 as a product. The oxygen is unbalanced, with
six atoms in the reactants and five in the products. Try 2 Pb(NO3)2,
which will give 2 PbO, 4 NO2, and two atoms of oxygen, making
one molecule of O2. Now the equation is balanced.
2 Pb(NO3)2(s)  2 PbO(s)
+ 4 NO2(g) + O2(g) 2 PbO(s)
+ 4 NO2(g) + O2(g)
|
C. Displacement Reactions
In displacement reactions, an uncombined element reacts with a compound and
displaces an element from that compound. For example, bromine is found in seawater
as sodium bromide. When chlorine is bubbled through seawater, bromine gas is
released and a solution of sodium chloride is formed:
2 NaBr(aq) + Cl2(g)  2NaCl(aq) + Br2(g)
2NaCl(aq) + Br2(g)
As another example, when an iron nail is dropped into a solution of copper(II) sulfate, iron(II) sulfate is formed in solution and metallic copper is deposited:
CuSO4(aq) + Fe(s)  FeSO4(aq) + Cu(s)
FeSO4(aq) + Cu(s)
Another displacement reaction, the reaction of metallic copper with silver nitrate, is shown in Figure 8.4.
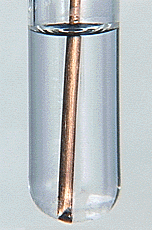

FIGURE 8.4 A displacement reaction. In the tube on the left
a copper wire has just been placed in a solution of silver nitrate. In
the tube on the right the reaction is almost complete, and a great deal
of silver has been deposited. The copper has displaced silver. The equation
for this reaction is 2 AgNO3 + Cu  Cu(NO3)2 + 2 Ag.
Cu(NO3)2 + 2 Ag. |
|
Example
Write balanced equations for the following displacement reactions::
a. When a piece of aluminum is dropped into hydrochloric acid hydrogen
is released as a gas and a solution of aluminum chloride is formed.
b. When chlorine is bubbled through a solution of sodium iodide crystals
of iodine appear in a solution of sodium chloride.
Solution
a. The unbalanced equation is:
Al(s) + HCl(aq) 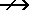 AlCl3(aq)
+ H2(g) AlCl3(aq)
+ H2(g)
Aluminum is balanced. To balance the chlorine, we need 3 HCl on the reactant
side, which will give 1 1/2 molecules of hydrogen.
Al(s) + 3 HCl(aq) 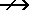 AlCl3(aq) + 1 1/2 H2(g)
AlCl3(aq) + 1 1/2 H2(g)
We need whole molecules of hydrogen. To get a whole number coefficient
without unbalancing the other elements, we can multiply the whole equation
by 2 to get:
2 Al(s) + 6 HCl(aq)  2 AlCl3(aq) + 3 H2(g)
2 AlCl3(aq) + 3 H2(g)
b. The unbalanced equation is:
Cl2(g) + NaI(aq) 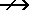 NaCl(aq)
+ I2(s) NaCl(aq)
+ I2(s)
to balance the chlorine, we must form 2 NaCl. Two NaCl require two sodium
atoms, or 2 NaI, which gives two iodine atoms, as needed. The balanced
equation then is:
Cl2(g) + 2NaI(aq)  2 NaCl(aq) + I2(s)
2 NaCl(aq) + I2(s)
|
Much more information is needed before you can predict whether or not a proposed
displacement will take place. That information is given in Chapter 14 (Oxidation-Reduction).
However, the displacement reactions we have discussed here will occur.
D. Double-Displacement Reactions
In
double displacement,
sometimes called metathesis or ion exchange, two ionic compounds react to form two different compounds. These reactions fall into a pattern that can be expressed as:
AB + CD  CB + AD
CB + AD
in which A and C are cations, B and D are anions. These reactions are often called "exchanging-partner" reactions because the cations A and C exchange the anions with which they are associated.
Double-displacement reactions fall into two categories: (1) those in which an acid reacts with a base to form a salt and water, which are known as neutralization reactions, and (2) those in which one of the products is insoluble, which are usually precipitation reactions, although occasionally the insoluble product is a gas.
1. Reaction of an acid
with a base: Neutralization reactions
In neutralization reactions, an acid reacts with a base to form a salt and water.
Recall from Section 5.7D that an acid is a
compound that liberates hydrogen ions in solution and a base (we will center
here on hydroxides, a subgroup of bases) is a compound that liberates hydroxide
ions in solution. A salt is defined as an ionic compound in which the cation
is not hydrogen and the anion is not hydroxide. These reactions are called neutralization
reactions because the base neutralizes the acid. Some examples are:
- The reaction of sodium hydroxide with hydrochloric acid to form sodium chloride and water:
NaOH(aq) + HCl(aq)  NaCl(aq) + H2O(l)
NaCl(aq) + H2O(l)
Note that the salt formed, sodium chloride, combines the cation of the base, Na+, with the anion of the acid, Cl-. The formula of the salt is the neutral combination of these ions, here a 1:1 combination in sodium chloride, NaCl.
- The reaction of magnesium hydroxide with phosphoric acid to form magnesium phosphate and water:
3 Mg(OH)2(aq) + 2 H3PO4(aq)
 Mg3(PO4)2(aq)
+ 6 H2O(l)
Mg3(PO4)2(aq)
+ 6 H2O(l)
Here again the salt formed, magnesium phosphate, Mg3(PO4)2, is a neutral combination of the cation of the base, Mg2+, with the anion of the acid, PO43- .
A
polyprotic acid
is one whose molecules ionize to yield more than one hydrogen ion. Sulfuric acid, H2SO4, phosphoric acid, H3PO4, and carbonic acid, H2CO3, are examples of polyprotic acids. When a polyprotic acid is one of the reactants, neutralization may be incomplete and an acid salt may form. An example of this reaction is:
NaOH(aq) + H2SO4(aq)  NaHSO4(aq) + H2O(l)
NaHSO4(aq) + H2O(l)
In this reaction, only one of the hydrogens of the diprotic acid reacted; the product is an acid salt, sodium hydrogen sulfate. The addition of more sodium hydroxide neutralizes the second hydrogen of this diprotic acid:
NaHSO4(aq) +NaOH(aq)  Na2SO4(aq) + H2O(l)
Na2SO4(aq) + H2O(l)
|
Example
Write balanced equations for the following neutralization reactions:
a. the complete reaction of sulfuric acid with calcium hydroxide
b. the complete reaction of magnesium hydroxide with hydrochloric acid
c. the reaction of sodium hydroxide with carbonic acid to form an acid
salt.
Solutions
a. The reactants are H2SO4 and Ca(OH)2. The products will show Ca2+ with
SO42- instead of with OH- and H+ with OH- (HOH is the same as H2O). Write
these facts in the form of an equation:
Ca(OH)2(aq) + H2SO4(aq) 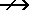 CaSO4(aq) + H2O(l)
CaSO4(aq) + H2O(l)
To balance the equation, note that there are two H+ and two OH-. They
will combine to give 2 H2O and the balanced equation:
Ca(OH)2(aq) + H2SO4(aq)  CaSO4(aq) + 2 H2O(l))
CaSO4(aq) + 2 H2O(l))
b. The formulas of the reactants are Mg(OH)2 and HCl. The products will
show Mg2+ and Cl- instead of OH- and H+ with OH- instead of Cl-. We write
the equation as:
Mg(OH)2(aq) + HCl(aq)  MgCl2(aq) + H2O(s)
MgCl2(aq) + H2O(s)
We need two chloride ions to combine with the Mg2+, so we write 2 HCl.
We now have 2 H+ and 2 OH- to combine with them to form 2 H2O:
Mg(OH)2(aq) + 2 HCl(aq)  MgCl2(aq) + 2 H2O(l)
MgCl2(aq) + 2 H2O(l)
c. The reactants are NaOH and H2CO3. If an acid salt is to be formed,
only one of the H+ in carbonic acid will be replaced. The cations changing
partners are Na+ and H+. The anions are HCO3- and OH-. Writing the equation
we get
NaOH(aq) + H2CO3(aq)  NaHCO3(aq) + H2O(l)
NaHCO3(aq) + H2O(l)
|
2. Double-displacement
reactions that form insoluble ionic products
Precipitation reactions, the second group of double-displacement reactions,
result in the formation of insoluble ionic compounds. Ionic compounds differ
enormously in the extent to which they dissolve in water, or their solubility.
Table 8.2 illustrates this point by listing the solubilities of several ionic
compounds in cold water. Notice that several, such as barium iodide and silver(I)
nitrate, are very soluble in water, whereas others, such as lead(II) chloride,
are only slightly soluble. Others, such as silver(I) chloride, are virtually
insoluble. Generally, if more than 0.1 g of an ionic solid dissolves in 100
mL (0.1 L) of water, the compound is said to be soluble. Less than 0.1 g calcium
carbonate, barium sulfate, and silver(I) chloride dissolve in 100 mL water.
Therefore, they are classified as insoluble compounds.

TABLE 8.2 Solubilities of ionic solids in cold water
| Name |
Formula |
Solubility
(g/0.1 L) |
| barium iodide |
BaI |
170 |
| silver(I) nitrate |
AgNO3 |
122 |
| sodium nitrate |
NaNO3 |
92.1 |
| ammonium chloride |
NH4Cl |
29.7 |
| lead(II) chloride |
PbCl2 |
0.99 |
| calcium carbonate |
CaCO3 |
1.4 X 10-3 |
| barium sulfate |
BaSO4 |
2.22 X 10-4 |
| silver(I) chloride |
AgCl |
8.9 X 10-5 |
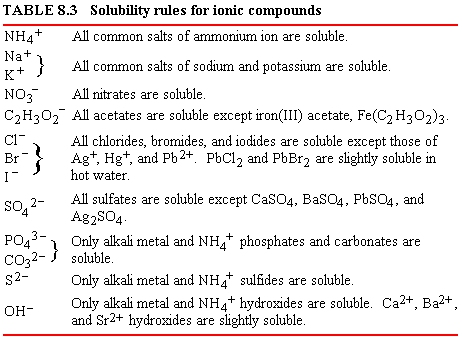
Table 8.3 lists some
solubility rules
by which the solubility of an ionic compound in water can be predicted.
|
Example
Write the formulas of the following salts and predict whether each is
soluble in water.
a. lead(II) nitrate b. iron(II) chloride c.
ammonium sulfide
d. barium sulfate
Solutions
| |
Formula |
Solubility |
Reason |
| lead(II) nitrate |
Pb(NO3)2 |
soluble |
It is a nitrate |
| iron(II) chloride |
FeCl2 |
soluble |
it is a chloride, but not one of the listed exceptions.
|
| ammonium sulfide |
(NH4)2S |
soluble |
It is an ammonium salt. |
| barium sulfate |
BaSO4 |
insoluble |
It is listed as an insoluble sulfate. |
|
In precipitation reactions, solutions of two ionic compounds are combined. If
two of the ions in the resulting mixture combine to form an insoluble compound
or precipitate, a reaction occurs. (Figure 8.5 shows the formation of a precipitate.)
If no insoluble product is produced, no reaction occurs.

| FIGURE 8.5 The formation of a precipitate. When a clear colorless solution of lead nitrate (Pb(NO3)2) is added to a clear colorless solution of sodium iodide (NaI), a yellow precipitate of lead iodide (PbI2) appears. The equation for this reaction is given in Example 8.7a. |
For example, if a solution of barium iodide is added to a solution of ammonium nitrate, no reaction takes place because the predicted products barium nitrate and ammonium iodide are both soluble.
BaI2(aq) + 2 NH4NO3(aq)
 Ba(NO3)2(aq)
+ NH4I(aq)
Ba(NO3)2(aq)
+ NH4I(aq)
A reaction would occur if a solution of barium iodide were added to a solution of silver nitrate, because one of the products, silver iodide, is insoluble.
BaI2(aq) + 2 AgNO3(aq)  Ba(NO3)2(aq) + 2 AgI(
Ba(NO3)2(aq) + 2 AgI( )
)
In the equations for these reactions, the physical state of the insoluble
product, the precipitate, is indicated either by (s) or by a downward-pointing
arrow after its formula; the soluble components of the reaction are shown
as (aq).
|
Example
Write the balanced equatio for the following reactions. Indicate with
a down-ward-pointing arrow any precipitate formed; name the precipitate.
a. Solutions of lead(II) nitrate and sodium iodide react to form a yellow
precipitate.
b. the reaction between a solution of copper(II) nitrate and a solution
of potassium sulfide yields a heay black precipitate.
Solutions
a. The formulas for the reactants are Pb(NO3)2 and NaI. The formulas
of the products of a reaction between these two compounds would have an
interchange of anions, yielding PbI2 and NaNO3.
Arranging these formlas in an unbalanced equation, we get:
Pb(NO3)2(aq) + NaI(aq)  PbI2 + NaNO3
PbI2 + NaNO3
Balancing this equation requires two iodide ions and therefore 2 NaI.
Two sodium nitrate are formed:
Pb(NO3)2(aq) + 2 NaI(aq)  PbI2(
PbI2(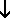 ) + 2 NaNO3(aq) ) + 2 NaNO3(aq)
Because all sodium salts are soluble, the precipitate must be lead(II)
iodide; we place an arrow after that formula.
b. The formulas of the reactants are Cu(NO3)2 and K2S. The formulas of
the products are CuS and KNO3. From Table 8.3 we know that potassium nitrate
is soluble, so the precipitate must be CuS, copper(II) sulfide. The unbalanced
equation is:
Cu(NO3)2(aq) + K2S(aq) 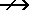 CuS{
CuS{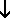 ) + KNO3(aq) ) + KNO3(aq)
Balancing this equation requires two potassium nitrate. The balanced
equation is:
Cu(NO3)2(aq) + K2S(aq)  CuS(
CuS(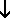 ) + 2 KNO3(aq) ) + 2 KNO3(aq)
All nitrates are soluble, so the precipitate is copper(II) sulfide. A
downward-pointing arrow is put after its formula.
|
As mentioned earlier, occasionally the insoluble product is a gas, as in the
following examples:
- Hydrogen chloride is released as a gas when concentrated sulfuric acid is added to solid sodium chloride. Although hydrogen chloride is very soluble in water, it is quite insoluble in concentrated sulfuric acid. The acid salt sodium hydrogen sulfate is the second product.
NaCl(s) + H2SO4(l) HCl(g) + NaHSO4(s)
HCl(g) + NaHSO4(s)
- Acetic acid may be released as a gas in a reaction similar to that in the first example. The equation for the reaction of concentrated hydrochloric acid with sodium acetate is:
NaC2H3O2(s) + HCl(aq)  HC2H3O2(g) + NaCl(s)
HC2H3O2(g) + NaCl(s)
- A carbonate reacts with an acid to form carbonic acid, which immediately decomposes to gaseous carbon dioxide and water.
Na2CO3(s) + 2 HCl(aq)  2NaCl(aq) + CO2(g) + H2O(l)
2NaCl(aq) + CO2(g) + H2O(l)