Applications
of Radioactivity
The use of radioisotopes is widespread in chemistry, biology, medicine, and other areas of science and industry. In all applications of radioactivity, the following characteristics of nuclear decay must be considered:
- The chemical properties and reactions of a radioisotope are exactly
the same as those of a non radioactive isotope of the same element.
- Radiation can be detected some distance from its source.
- Each radioisotope has a characteristic half-life.
- Radioactive emissions interfere with normal cell growth.
Several uses of radioisotopes in the health and biological sciences illustrate
ways in which they can provide information that would be difficult or impossible
to obtain by any other means.
A. The Use of Tracers
Because the chemical properties and reactions of a radioisotope are exactly the same as those of a nonradioactive isotope of the same element, a radioisotope can be substituted for a stable isotope of the same element in a molecule or compound, without changing the chemical properties of the compound. Such a compound is said to be "tagged" or "labeled." Because the radiation emitted by the radioisotope can be detected some distance from the radiating atom, the progress of those atoms through the body can be followed, or traced. Hence, such labeled compounds are called
tracers.
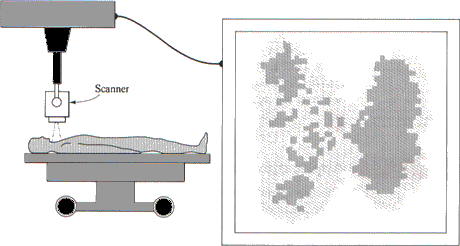
Iodine-131 is the most commonly used radioisotope for the study of iodine metabolism in humans. The thyroid gland has a remarkable ability to extract iodine from the bloodstream and use it to produce the thyroid hormones, thyroxine and triiodothyronine. These two hormones have a direct effect on the body's metabolism. Very small amounts of iodine-131, in the form of sodium iodide, can be injected into the bloodstream and, within minutes, will begin to concentrate in the thyroid gland. By monitoring the accumulation of radioactivity, it is possible to estimate the size and shape of the thyroid gland and to determine whether any part of it is functioning abnormally (Figure 4.4).
|
FIGURE 4.4 The uptake of iodine by the thyroid gland can be measured by tracing atoms of radioisotope iodine-131. The plot at the right of the figure shows the location in the thyroid of the source of each radioactive emission detected by the counter. Note that the radioactivity counts show more iodine in one lobe of the thyroid gland than in the other.
|
Several other radioisotopes commonly used in nuclear medicine are listed in
Table 4.7. Notice that all have comparatively short half-lives and that all
are pure gamma or beta-gamma emitters.
TABLE 4.7 Radioisotopes used in nuclear medicine for clinical diagnosis
| Radiation |
Half-life |
Used to study |
| Radioisotope |
Particle emitted |
| chromium-51 |
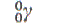 |
27.8 days |
red blood cells |
| cobalt-57 |
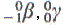 |
270 days |
absorption, storage, and
metabolism of vitamin B-12 |
| iron-59 |
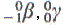 |
45.1 days |
red blood cells |
| strontium-87 |
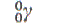 |
2.8 hours |
bone metabolism |
B. Biological Effects of Radiation
Although the exact manner in which radiation damages tissues and cells is not
fully understood, cellular damage clearly can occur any time alpha or beta particles
or other ionizing radiation passes through the cell. The effects of ionizing
radiation range from minor damage of specific molecules to the death of cells.
Ionizing radiation is especially damaging to the cell nucleus - particularly
nuclei undergoing rapid division and nuclei of younger, less mature cells. Many
types of cancer cells are especially sensitive to gamma radiation because they
are growing rapidly and are therefore less mature than cells of surrounding
noncancerous tissue. This sensitivity is the reason behind the use of radiation
to destroy cancer cells. The apparatus used for such treatments is designed
to focus the radiation sharply on the cancer cells, thus minimizing the damage
to nearby healthy cells.