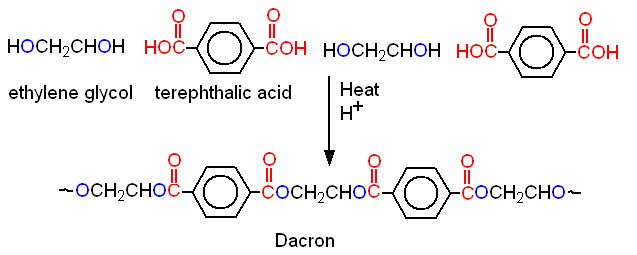
Suppose an esterification reaction is carried out between an acid that contains two carboxyl groups and an alcohol with two hydroxyl groups. The result will be a polymer, a polyester. Since each time an ester bond is formed, a molecule of water is produced, polymerization of this type is called condensation polymerization to distinguish it from addition polymerization of alkenes, which we studied in "Polymers." Dacron, the best known example of a polyester, is formed by the loss of water between a dicarboxylic acid, terephthalic acid, and a diol, ethylene glycol (Fig. 12-15)

Figure 12-15. Dacron is a polyester, formed by the condensation polymerization of a diol and a diacid.
Dacron is a linear polymer that usually contains about 80 units per chain, a solid which can be melted without decomposition. The molten polymer is forced through tiny holes in a spinneret and cools and solidifies in the form of thin fibers. These fibers are then stretched to about five times their original length. During the stretching process the individual polymer molecules become aligned so that they all lie parallel to the fiber axis; this allows the molecules to pack closely together, making the yarn stronger. Closer packing of the polymer molecules also makes it more difficult for other molecules to penetrate the fiber, and drawn Dacron is more chemically resistant and absorbs less water than the undrawn polymer. For the same reason, it is difficult to dye Dacron, since the dye molecules cannot find room between the polyester chains. In the form of a film, this same polyester, polyethylene terephthalate, is known commercially as Mylar. In Europe, this polyester is called Terylene, and the fibers Fortrel and Kodel made in the United States have similar structures. Still other polyesters are prepared from aliphatic diacids.
Formation of a polyester from a triol instead of a diol can give rise to a polymer that is not linear like Dacron but rather highly cross-linked and three-dimensional. Common members of this class are the glyptals, made from glycerol and phthalic acid, the isomer of terephthalic acid in which the carboxylic acid groups are ortho instead of para to one another.
Copyright (c) 1999. All rights reserved.