 |
Chemistry
234
|
Experiment 4: Extraction (20 points)
Summary
Extraction is a technique used to separate and/or purify compounds. In this experiment, you will be separating a mixture of anthracene, p-nitroaniline, and benzoic acid into the individual components based on their differing solubilities in immiscible phases.
|
Mixture of anthracene, p-nitroaniline, and benzoic acid. |
 |
| Purified benzoic acid |  |
| Purified anthracene |  |
| Purified p-nitroaniline |  |
Prelab
BEFORE class, prepare a flow chart that outlines every step of the experiment. The flow chart should show a branch point at every extraction step (including isolation of the three compounds), and you should note which compounds are in each layer at each step. You must have this flow chart completed before you will be allowed to begin the experiment,and the flow chart will be handed in with your lab report. Examples of flow charts are on pp. 148-149 of your text book.
Procedure
Be sure to wear rubber gloves when doing this experiment.
Obtain a mixture of benzoic acid, p-nitroaniline, and anthracene. Weigh the mixture, and then dissolve the mixture in 75 mI, of methylene chloride. If some solid doesn't dissolve after several minutes of stirring, filter the solution. Add the solution to the separatory funnel and proceed through the following steps:
First Period:
1 . Add 50 mL of 3M HCI to the separatory funnel. Shake and vent the funnel as described in the textbook readings.
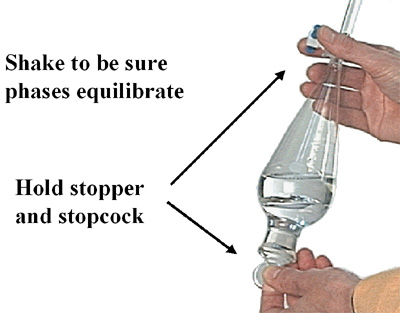
Be sure to vent the separatory funnel to avoid pressure build up. If you get hydrochloric acid on your skin be sure to wash with large amounts of water. Contact your TA for assistance.
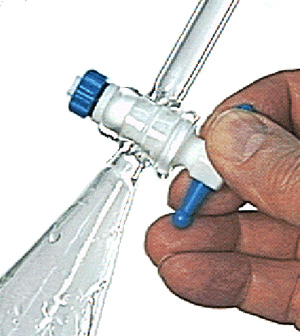
2. Allow the layers to separate in the funnel. Remember to remove the stopper before draining. Drain the bottom layer into a flask labeled "organic," and pour the top layer into a flask labeled "acidic extract"

3. Return the organic layer to the separatory funnel, and repeat steps 1 and 2. Add the top layer from this extraction to the flask labeled "acidic extract".
4. Place the organic extract into the separatory funnel, and add 50 mL of 3M NaOH. Shake and vent the separatory funnel as before.
If you get sodium hydroxide solution on your skin be sure to wash with large amounts of water. Contact your TA for help.
5. Allow the layers to separate. Drain the bottom layer into a flask labeled "organic," and pour the top layer into a flask labeled "basic extract."
6. Return the organic layer to the separatory funnel, and repeat steps 4 and 5. Add the top layer from this extraction to the flask labeled "basic extract."
7. Cool the ACIDIC EXTRACT in an ice bath. Basify the ACIDIC EXTRACT with 6M NaOH (calculate how much you need). Check the pH of the solution with pH paper to make sure it is BASIC. Filter the resulting precipitate by vacuum filtration. Place the solid on a piece of filter paper, and allow it to air dry until the next lab period.
USE CAUTION IN HANDLING THE SODIUM HYDROXIDE.
8. Cool the BASIC EXTRACT in an ice bath. Acidify the BASIC EXTRACT with 6M HCl. Check the pH of the solution with pH paper to make sure it is acidic. Collect the resulting solid by vacuum filtration. Place the solid on a piece of filter paper and allow it to air dry until the next lab period.

9. To the ORGANIC layer, add a few spatula tips of Na2SO4 to dry the organic layer. Allow the solution to stand for 10 minutes.
10. Filter the solution through fluted filter paper into an Erlenmeyer flask. Evaporate the methylene chloride using the apparatus that will be demonstrated in lab.

Second Period:
1. Obtain weights of benzoic acid, p-nitroaniline, and anthracene and melting points of benzoic acid and p-nitroaniline.
2. Place all solids in tared, labeled vials, and give them to your TA.

Report Requirements
Your report should contain the following:
I . Reference to procedure with changes noted
2. Flow chart described above. Draw structures of all species. Show ionic structures were appropriate.
3. Weights of all three solids. Descripte their physical properties.
4. Melting points of benzoic acid and p-nitroaniline (both weights and melting points should be reported in tabular form).
5. Describe the solids
6. Interpretation of data and conclusion
-How pure are the extracted compounds?
-How could you purify them further?
-What was the composition of the original mixture, i.e., the ratio of benzoic acid: p-nitroaniline: anthracene?
- recovery
Answer these questions in your report:
1. If you have 10 grams of A in 100 mL of water and extract 3 times with 50 mL of ether what is the total yield of A in the combined ether solutions? Assume the distrbution coefficient for A is 2.0. Show all of your work.
2. What physical property determines which layer is on the top in an extraction?
3. How would you extract N,N-dimethylaniline from methylene chloride into water?
4. How would you extract p-nitrobenzoic acid from methylene chloride into water?