 |
Chemistry
234
|
Experiment 10: Identification of an Aldehyde, Amine, Alcohol, or Ketone (45 points)
I. Summary
In this experiment you will be given an unknown that is either an aldehyde, ketone, amine or alcohol. The possible unknowns are listed in tables in the lab. You will be given IR and NMR spectra to help you determine the functional group and structure of your compound. You will also obtain the boiling point or melting point of your compound, perform classification tests, and make a derivative.
II. Procedure
The week before this experiment begins, you will receive IR, 1H NMR, and 13C NMR spectra of your compound.BEFORE the first day of this experiment, make a tentative functional group assignment. You do not need to determine the structure of the unknown before class, but you will need to analyze you spectra for structural information at some point during the course of the experiment. During the class periods assigned for this experiment, do the following in the order listed:
1. Distill your unknown, if it is a liquid, to obtain a boiling point of the pure compound. |
To obtain a boiling (or melting) point range, subtract 10°C from the observed temperature to get a lower limit, and add 10° to the observed temperature to get an upper limit.
2. Perform all three of the following classification tests.
You should first perform these tests on the provided known compounds for comparison.
|
 |
|
|
 |
C. Test for pH - If your compound is water-soluble, use pH paper to test an aqueous solution of your unknown. An amine, since it is basic, will have a high pH. If your unknown is not water-soluble, dissolve the unknown in an ethanol-water mixture, and test the pH.
3. Make a derivative
Once you have determined the functional group present in
in your unknown, choose the appropriate derivative from the list below:
Aldehyde/ketone: 2,4-DNP (page 765-766)
Alcohol: 3,5-dinitrobenzoate (page 780-781, Method A, see Note 1 for amounts of
reagents)
Amine: benzenesulfonamide (page 793-794) or benzamide.
III. Lab Report Requirements
Your lab report (due the day scheduled for check-out) should contain the following:
1. Reference to the procedure of EACH chemical test and the derivative
2. Melting/boiling point of the unknown
3. Results of each chemical test and your interpretation of the results. Write chemical equations for the tests.
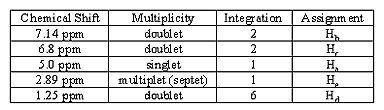
4. Data table for 1H NMR (see example)
5. Data table for 13C NMR: For each relevant peak, report the chemical shift and assignment.
6. Data table for IR (assign bands above 1500 cm-1): For each band, report position (in wavenumbers), a description of the band (strong, medium, or weak; sharp or broad), and your interpretation
7. Type of derivative and the melting point (include the reference melting point). Write chemical equations for the formation of each derivative.
8. Identification of the unknown with a THOROUGH explanation of how you arrived at this conclusion. Draw the STRUCTURE of your unknown.
Notes:
1. Preparation of the 3,5-dinitrobenzoate derivative (amounts):
0.5g 3,5-dinitrobenzoylchloride (this will be preweighed and available from
the storeroom)
1.5mL of your unknown alcohol
20 drops of pyridine (Caution! Pyridine should be used in the fume hood
since it has a VERY unpleasant odor. Do not pour pyridine or
filtrates containing pyridine down the drain)
EXAMPLE 1H data writeup
Spectrum
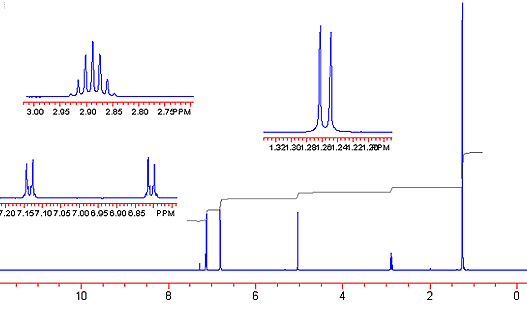
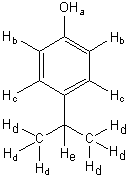
Make a table that shows chemical shift, multiplicity, and integration area for each of your protons.