 |
Chemistry
234
|
Experiment
7:
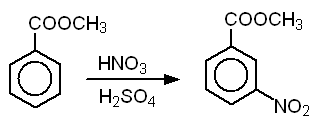
Preparation of methyl-m-nitrobenzoate (25 points)
I. Summary
In this experiment, you will synthesize methyl-m-nitrobenzoate from methyl benzoate via electrophilic aromatic substitution. The isolated product will be purified by recrystallization, and purity will be determined from the melting point.
II. Procedure
Bring a clean, dry 25 x 150 mm test tube to the storeroom manager and trade it in for a 6.2 mL (6.8 g, 0.050 moles) sample of methyl benzoate (weigh the sample before you begin to obtain an exact weight). Place 14.5 mL (26.7 g) of concentrated sulfuric acid in a 125 rnL Erlenmeyer flask, cool it to 0 °C in an ice/water bath, then add all of the methyl benzoate with swirling.
Separately, prepare a mixture of 5.0 ml- (9.2 g, 0.094 moles) of concentrated sulfuric acid and 5.0 mL (7.1 g, 0. 113 moles) of concentrated nitric acid.
Cool the nitric acid in an ice bath and then very slowly add the sulfuric acid keeping the mixture cold.Be very careful in handling the concentrated sulfuric acid and nitric acid. If you get acid on you be sure to wash with large amounts of water and contact your TA immediately for assistance.
| When it has cooled, add this cold acid mixture dropwise over 5 minutes to the methyl benzoate solution, which is kept swirling in the ice bath. |  |
|
After the addition is complete, allow the reaction mixture to stand at room temperature for an additional 10 minutes, swirling it occasionally. |
 |
|
Pour the mixture, with stirring, over about 150 cc of crushed ice. Collect the solid precipitate by suction filtration, and wash it thoroughly with water to remove any traces of acid. |
 |
|
Recrystallize your product from a small amount of methanol. Collect the crystals by vacuum filtration and wash with a small amount of COLD methanol. |
 |
Allow the product to air dry in your drawer until the next lab period. Obtain the weight and melting point of your dry product. Give your sample to your TA.
III. Report Requirements
Your report should contain the following information:
1. Reference to the procedure with changes noted
2. Equation of the reaction showing structures of reactants and products. Below each reactant, list the molecular weight, and amount used in grams (or mLs) and moles. Below each product, list the theoretical yield in grams and moles
3. Mechanism of the reactions showing the structures of intermediates and arrows to demonstrate electron movement
4. Experimental yield and percent yield (show calculations)
5. Color and physical properties of your product
6. Melting point of product
7. Give chemical and physical reasons for not obtaining 100% yield. Write equations for side reactions and explain how side products were removed from the product.
8. Interpretation of data and conclusion.
9. Answer these questions in your report:
- Draw all resonance structures for the nitration of methyl benzoate occurring at the ortho, meta, and para positions. Be sure to include all charges.
- Circle any resonance structures that demonstrate why the ortho-para positions are unfavorable sites for nitration.