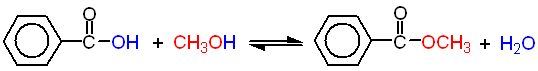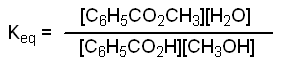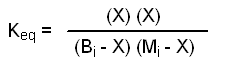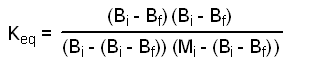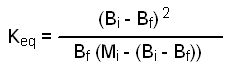 |
Chemistry
234
|
Experiment
6: Preparation
of methyl benzoate
(40 points)
I. Summary
In this experiment you will prepare methyl benzoate by reacting benzoic acid with methanol using sulfuric acid as a catalyst. Since this is a reversible reaction, it will reach an equilibrium that is described by the equilibrium constant, Keq .
In this experiment, you will isolate the product, methyl benzoate and any unreacted benzoic acid. Using this data, you will calculate the equilibrium constant for the reaction as described in part III.
II. Procedure
First Period
|
Obtain a 10g (0.082 mol) sample of benzoic acid from the storeroom (weigh the benzoic acid to obtain an exact weight before you begin). Add to it 0.62 mol of methanol in a 100 mL round-bottomed flask. Cautiously, add 3 mL concentrated H2S04 down the side of the flask. After gently swirling the contents of the flask, attach a reflux condenser and reflux the mixture for about 60 min. Sulfuric acid causes acid burns on the skin. If you get some on your skin be sure to wash with large amounts of water. Contact your TA! |
 |
Allow the solution to cool. Add 50 mL of water to a separatory funnel, then add the contents of the flask. The flask should then be washed with 40 mL of dichloromethane (CH2Cl2) and the washings transferred to the separatory funnel. Use the separatory funnel to extract the ester into the CH2Cl2 (organic) layer. Wash
sequentially with 25 mL water and 25 mL 0.6M sodium bicarbonate* (Caution, CO2 will be given off with effervescence).After shaking and venting the funnel, remove the sodium bicarbonate layer and test to see if it is basic. Then wash the organic layer with NaCl (salt) solution, separate the organic layer and dry with anhydrous magnesium sulfate. Remove the MgS04 by gravity filtration, and concentrate the solution using the same apparatus used to remove CH2Cl2 from anthracene in Experiment 4.
*The aqueous layer from the sodium bicarbonate wash should be acidified with concentrated HCl The unreacted benzoic acid should precipitate. Remove the solvent by vacuum filtration. Collect and dry the solid benzoic acid and let it air-dry in your drawer until the next lab period.
Second Period
Distill the crude methyl benzoate (b.p. 199°), collecting anything that boils between 170°C and 200°C. Weigh the sample of methyl benzoate and determine the yield. Weigh the sample of recovered benzoic acid. Place both samples in labeled vials, and give them to your TA.
Put aluminum foil around your flask for insulation because of the high boiling point of methyl benzoate.

Caution:
1. Methanol is flammable and toxic
2. Dichloromethane is toxic3. Handle concentrated H2SO4 and HCl with great care
4. Vent the separatory funnel frequently after swirling and subsequent shaking. CO2, gas is evolved and will build up pressure in the funnel.
III. Calculation of the Equilibrium Constant, Keq.
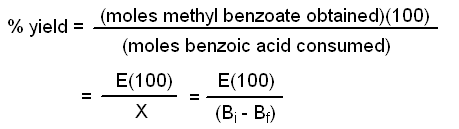
Calculate a value for the equilibrium constant Keq based upon your weight of recovered benzoic acid. In addition, calculate the percent yield of your synthesis based upon the amount of starting benzoic acid used, and another percent yield based upon the amount of benzoic acid that actually reacted (as determined by the weight of recovered benzoic acid).
Calculation of the Equilibrium Constant Keq:
Since the volume of the reaction mixture is the same for each component, we can use moles instead of molarities as the units in our Keq expression. Let Bi = initial moles of benzoic acid, let Mi = initial moles of methanol, and let X = final moles of methyl benzoate produced at equilibrium.
Based upon the stoichiometry of equation 1, we can conclude three things about X, namely that X = final moles of water produced at equilibrium, X = moles of benzoic acid consumed when equilibrium is established, and X = moles of methanol consumed when equilibrium is established. Using our definitions, equation 2 becomes:
We know Bi and Mi. All we need to do before we can calculate Keq is to Put X in terms of something known, like the amount of benzoic acid recovered. Let Bf = final moles of benzoic acid present at equilibrium. Thus Bf = Bi - X, or X = Bi - Bf. Substituting(Bi - Bf) for X in equation 3 gives:
We can now calculate Keq by entering the appropriate values of the moles of benzoic acid used (Bi), the moles of methanol used (Mi), and the moles of benzoic acid recovered (Bf) into equation 5.
Calculation of the % Yield based upon initial amount of benzoic acid used:
Let E = moles of methyl benzoate ester obtained. Note that this calculation is valid only if benzoic acid is the limiting reagent.
Calculation of the % Yield based upon amount of benzoic acid that is consumed:
IV. Report Requirements
Your lab report should contain the following information:
1 . Reference to procedure with any changes noted
2. Equation of the reaction showing structures of reactants and products. Below each reactant, list the molecular weight, and amount used in grams (or mLs) and moles. Below each product, list the theoretical yield in grams and moles.
3. The complete mechanism showing all intermediates and arrows to demonstrate electron movement
4. Flow chart for the isolation of methyl benzoate and unreacted benzoic acid
5. Weight of recovered benzoic acid
6. Weight and boiling point of methyl benzoate
7. Calculations
a. percent yield based on amount of benzoic acid with which you started
b. percent yield based on amount of benzoic acid consumed
c. Calculate the equilibrium constant.
8. Interpretation of data and conclusion. Explain what was done in this experiment to drive the reaction toward ester formation.
9. Answer these questions in your report:
1. How is sulfuric acid involved in the formation of methyl benzoate?
2. Write a balanced equation for the formation of ethyl acetate from ethanol and acetic acid.
3. Using K=3.0, calculate the number of moles of methyl benzoate which could be formed from 0.1 mol of benzoic acid and 0.3 mol of methanol.