 |
Chemistry
234
|
Experiment
9:
Preparation
of trans-p-Anisalacetophenone
(25 points)
I. Summary
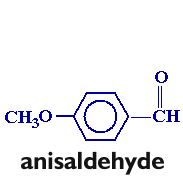
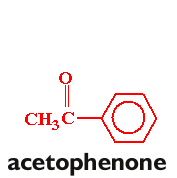
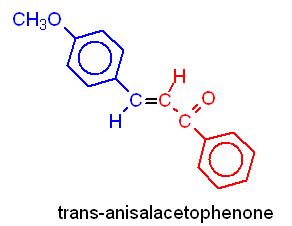
In this experiment, you will be preparing trans-p-anisalacetophenone (m.p. 77-78°) from p-anisaldehyde and acetophenone via a crossed aldol condensation. The product will be purified by recrystallization, and purity will be assessed by taking the melting point of the product.
 |
 |
 |
II. Procedure
Follow the procedure found on pages 572-576 in your text (omit the "Analysis" portion of the procedure. You will need to scale the procedure to six times that listed in the text. Take a clean, dry 25 x 150 mm. test tube to the storeroom manager and trade it in for a sample of p-anisaldehyde. Weigh the sample before you begin the experiment, and adjust the amount of acetophenone accordingly. Once you have obtained the recrystallized product, allow the product to air-dry in your drawer until the next lab period. Obtain the weight and melting point.
|
Locate starting material |
 |
|
Measure reactants |
 |
|
Mix |
 |
|
Let stand
|
 |
| Cool |  |
|
Filter crude product |
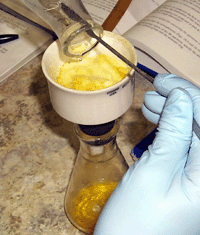 |
|
Recrystallize
|
 |
|
Purified product |
 |
III. Report Requirements
Your report should contain the following:
1. Reference to procedure with changes noted.
2. Equation of the reaction showing structures of reactants and products. Below each reactant, list the molecular weight, and amount used in grams (or mLs) and moles. Below each product, list the theoretical yield in grams and moles.
3. Mechanism of the reactions showing the structures of intermediates and arrows to demonstrate electron movement
4. Mass of product and physical properties
5. Percent yield (show calculations)
6. Melting point of product (include literature melting point)
7. Interpretation of data and conclusion, including detailed error analysis
8. Answer these questions in your report:
1. Write the mechanism for the condensation of acetophenone with itself.
2. Write the mechanism for the Cannizzaro reaction of anisaldehyde.3. Why is the product trans-p-anisalacetophone instead of cis?