 |
Chemistry
234
|
Experiment 5: Preparation of 2-chloro-2-methylpropane (20 points)
Summary
In the experiment, you will be preparing 2-chloro-2-methylpropane (tert-butyl chloride) from 2-methyl-2-propanol (tert-butyl alcohol) via an SN1 reaction.
![]()
Procedure
You will follow the procedure from the text (pages 435-439). Note that you will be using 2-methyl-2-propanol instead of 1-methyl-2-butanol. However, all other reagents remain the same. You should double all quantities listed in the procedure.
|
Shake the t-butyl alcohol with HCl. Be sure to vent the separately funnel to avoid pressure build up. If you get hydrochloric acid on your skin wash immediately with large amounts of water. Contact your TA for assistance.
Separate, wash, and dry the t-butyl chloride. |
 |
|
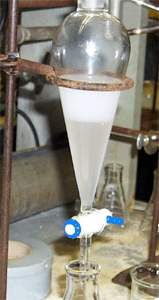
The crude t-butyl chloride is washed to remove excess acid. Add the sodium bicarbonate solution slowly to avoid rapid formation of CO2 as shown here. |
 |
|
Setup for distillation of the t-butyl chloride. Collect the product in a cooled, tared container. |
 |
|
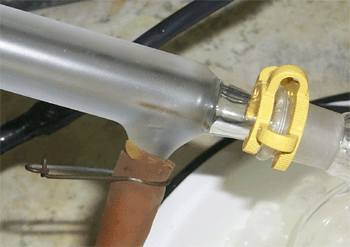
Use joint clamps on the condenser and hose clamps on the water lines. |
 |
Report Requirements
Your report should contain the following:
1 . Reference to the procedure with changes noted
2. Equation of the reaction showing structures of reactants and products. Below each reactant, list the molecular weight, and amount used in grams (or mLs) and moles. Below each product, list the theoretical yield in grams and moles. Give the density and boiling point of liquids.
3. Mechanism of the reaction: use arrows to show electron movement; include all intermediates and side reactions
4. Calculation of the theoretical yield
5. Weight of product and description including color
6. Boiling range of the product
7. Interpretation of data and conclusion. Give chemical and physical reasons for not obtaining a 100% yield.
8. Answer the following questions in your report.
1. Washing the crude t-butyl chloride with aqueous sodium bicarbonate resulted in gas evolution. Write a balanced equation for this reaction.
3. Write the mechanism for the reaction of n-butyl alcohol with HCl and compare it to the reaction of t-butyl alcohol with HCl.