Visible Light Spectroscopy
Preparation
Procedure
|
The Hydrogen Atomic Spectrum
|
|
Proceed to a station with a hydrogen discharge tube in the
power supply. Look at the hydrogen discharge tube with your
spectroscope just as you did for the helium discharge tube.
Again, although both group members should view the spectrum,
only one should take data in order to be consistent. Make
sure that your eye position is consistent relative to the
eyepiece.
|
 |
|
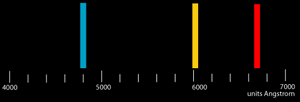
Describe what you see in the spectroscope. Include the number
of lines, the color and the wavelength of each line. The fourth
line for hydrogen may or may not be visible; its actual wavelength
is 4104 Angstroms. Record the experimental wavelength for
the fourth line if it is visible.
|
 |
| |
| |
|
|
|
|
|
|