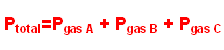Introduction |
|||||||||||||||||||||||||||||
“Mr. Zippo”, also known as George G. Blaisdell, invented the Zippo™ lighter in 1932. Like many others, he was looking for financial stability during the Depression. Originally, he patterned it after an Austrian lighter, improving the appearance, but these didn’t sell. He tried again, this time making it smaller, adding a hinged lid, using what’s called a “ wind hook” around the wick, and marketing the Zippo™ with the first lifetime guarantee. It sold for $1.95. Since then, these lighters have become extremely popular. Perhaps it is because of their resilience and utility. (One story is told of a Zippo™ that lit on the first try—after being removed from a fish.) Zippo’s TM are especially known for their utility in war. Soldiers have carried them since World War II, using them for everything from signaling helicopters to storing salt that would replenish what was lost sweating*. Zippo™ lighters are different from everyday, plastic lighters because they contain lighter fluid, not butane. A plastic lighter like the ones you will be using today contains only butane, C4 H10. Why use butane to study gasses and gas laws? First of all, butane is easily collected, as we will show today. Most important, though, is that butane is close to “ideal” at standard temperature and pressure. Ideal gasses are described by the ideal gas law, which states that the product of the pressure and volume of a gas is proportional to the product of the number of moles and the Kelvin temperature. Emil Clapeyron first wrote this in 1834, and we’ll write it again here. R is the gas constant. The value depends on the units used. When pressure is reported in atmospheres (atm), volume in liters (L), and temperature in Kelvin (K), the gas constant has a value of:
You will use the ideal gas law today to find the molar mass of butane. Although butane can be described by the ideal gas law, it is important to remember that it is not ideal. Later on you will use corrections to the ideal gas law to see how butane’s behavior deviates from ideal gas behavior. To find the molar mass of butane, you will collect butane gas by releasing it from a lighter and collecting it over water in a graduated cylinder. You will be able to find the volume of gas released in this way. In addition, you will make measurements of both temperature and pressure. With the volume, temperature, and pressure of butane, you can use the ideal gas law to find the moles of butane released from the lighter. While measuring volume and temperature is accomplished easily, measuring the pressure of the butane gas in the graduated cylinder is more complicated. The relevant gas law which will help you to do this is Dalton’s Law of Partial Pressures. It states that a gas exerts a certain pressure regardless of the presence of other gasses. This means that calculating the pressure of each gas in a mixture independently and summing these individual pressures determines the total pressure.
Or in our case, since we are collecting the gas over water,
Water vapor pressure (Pwater) will depend on temperature. Since water is a factor in so many experiments, charts that provide pressure values at common temperatures are readily available (though charts for other gasses can be obtained as well). Such a chart is provided here.
*About Zippo Lighters. http://www.zippo.com/main.aspx |
|||||||||||||||||||||||||||||