Describe the Products of Several Reactions |
|
| Here you'll look at the results of each combination of two reagents. Take careful observations! Note color changes, formation of precipitates, and formation of gasses (indicated by bubbles). Rushing through this part will make your unknown determination harder. Of course, feel free to experiment. Add another drop of one or both reagents if you can't see anything at first, and don't forget to stir. | |
|
Helpful hints:
|
|
| Before you run any reactions, record your observations about the compounds themselves (these may be very short). | |
| Mix the reagents in pairs according to the reaction chart. | |
| Record your observations on the observations chart. | 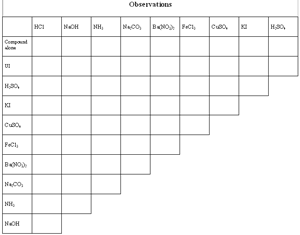
|