Deduce the Identity of Unknown Compounds |
|
|
In this portion of the lab you will have four unknowns to identify. Two will be one of the compounds you've already worked with, one will contain an anion you've worked with, and another a cation you've worked with in this lab.
|
|
|
Set up another reaction chart. This time use the unknowns chart. It has a lot of room for you to experiment. |
|
|
You will also want to label the wells you keep your unknown microburettes in, as well as the microburettes themselves. |
|
|
Observe the color of your unknowns. |
 |
|
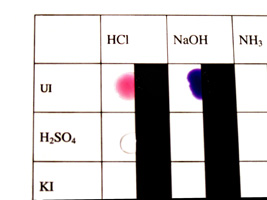
Test each unknown with the Universal Indicator. |
|
|
Mix each unknown with your known compounds. |
|
|
Binary mix your unknowns with each other. |
|
|
You may want to redo some of the known reactions for comparison. |
|
|
When you think you know the identity of your compounds, show your results to your TA. |
|